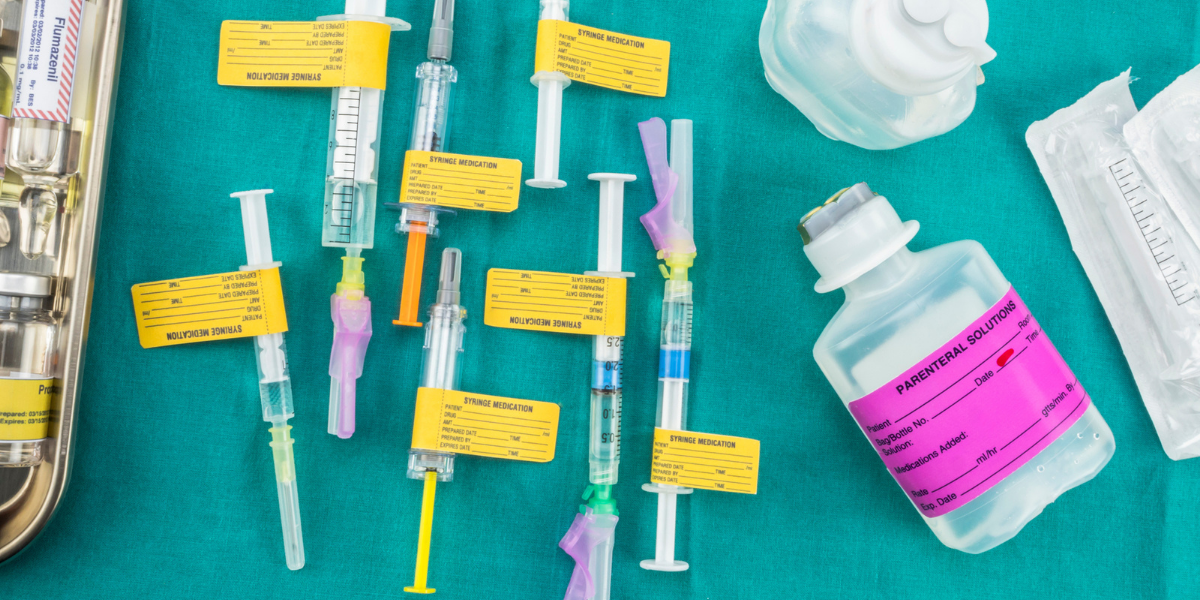
Using Medical Device Labels Without Complications
Medical device labeling can be more complicated than you would imagine. For example, not many businesses know that you cannot just copy a label from an existing device. Furthermore, the label is not limited to just the actual label. It must additionally incorporate the instructions for use (IFU), medical components, ingredients, details, and more.
Let us learn more about medical device labels to avoid confusion.
Medical device Labels include the instructions for use and more
It is not just the label. Medical device labels include (as per the FDA):
- Instructions for use (IFU).
- Posters, tags, pamphlets, and circulars.
- Intended accompanying articles such as booklets, brochures, instruction books, direction sheets, fillers, and so on.
- Technical descriptions, details for the proper use of the medical device, etc.
All the accompanying material (accompanying in not just a physical sense) for a medical device falls within medical device labeling except the shipping documents.
Printing the Correct Medical Device Labels
Getting the medical device labels right is the #1 prerequisite to getting FDA approval. You cannot cut corners here. You need to do your due diligence in making sure that even a nonprofessional can understand the basic usage, application, and technical directions for the medical device.
It has to be clear, appropriately bold, and uncluttered. Anything that interferes with the visibility is a big no-no. The colors, fonts, spacing, layout, and any iconography has to be as minimal yet comfortably understandable as possible.
Users rely heavily on the medical device labels and it’s your responsibility to make it as clear and easy to understand as possible, both technically and visually.
Risk Management with Medical Device Labels
Medical devices come with various risks. If you take predicate device labeling and simply replace the name with yours, it will not do.
A predicate label is a good way to start. You will know the common warnings and cautions to incorporate. However, that is only half the work.
Your design is unique. As such, its working is also slightly different from the predicate device. The hazardous situations and harms that you have identified for your device must exist on the medical device label.
It also happens to be true that many predicate devices were created and designed years ago. The medical world goes through major breakthroughs and innovations monthly. As such, many cautions and warnings can now be outdated.
Risk mitigation is a critical part of designing and manufacturing a new medical device. And medical device labeling is perhaps the single most important method to do it effectively.
Effective medical device labeling
There are plenty of options to choose from. The entire procedure requires a reliable medical label printer, label materials, label design software (label management software), and a little bit of testing.
Follow the industry standards, learn from the best, and do your due diligence. Think from the point of view of your customer and test extensively in multiple scenarios.